Clinical Labs
Join the SalivaDirect, Inc. Designated Lab Network!
SalivaDirect™ is a PCR test that uses saliva to detect SARS-CoV-2 in patients two (2) years and older. Our flexible, extraction-free method is designed to be a cost-effective, simple test for laboratories, enabling them to achieve higher throughput and faster results.
The U.S. FDA granted the SalivaDirect™ EUA (EUA 202097) in August 2020 and authorized SalivaDirect, Inc.* to extend the EUA to designated CLIA laboratories across the country. SalivaDirect, Inc. has designated more than 200 labs in 42 states. High-complexity laboratories in the U.S. can be designated to perform the SalivaDirect™ test.
Qualified labs are invited to join our network. With documents in hand, most labs complete the designation process within one week.
*The EUA was initially authorized to designate labs under Yale School of Public Health.
Laboratory Designation Process
Clinical laboratories interested in being designated to offer SalivaDirect™ as part of their laboratory services must apply to join the network. SalivaDirect, Inc. will review each application to determine eligibility. If your lab is eligible to join the network, you will complete the following steps:
Perform the SalivaDirect™ protocol using the Instructions for Use
Provide testing verification data demonstrating mastery of the SalivaDirect™ protocol
Complete the Designated Laboratory Agreement
Receive access to training and documents for the SalivaDirect™ protocol in our Lab Portal
The SalivaDirect™ Protocol
The SalivaDirect™ protocol is authorized for emergency use by the U.S. Food and Drug Administration to diagnose COVID-19 in both symptomatic and asymptomatic individuals.
The SalivaDirect™ protocol is one of the first diagnostic approaches to offer reagent and instrument-flexible capabilities, allowing labs to use several commercially available reagents and instruments from different suppliers. Validated across a wide range of reagents and instruments, the protocol can be seamlessly integrated into many existing lab setups reducing the barrier to entry for labs to begin providing COVID-19 diagnostic testing.
Testing is limited to laboratories designated by SalivaDirect, Inc. that are certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, and meet the requirements to perform high-complexity tests.
How Does the SalivaDirect™ Protocol Work?
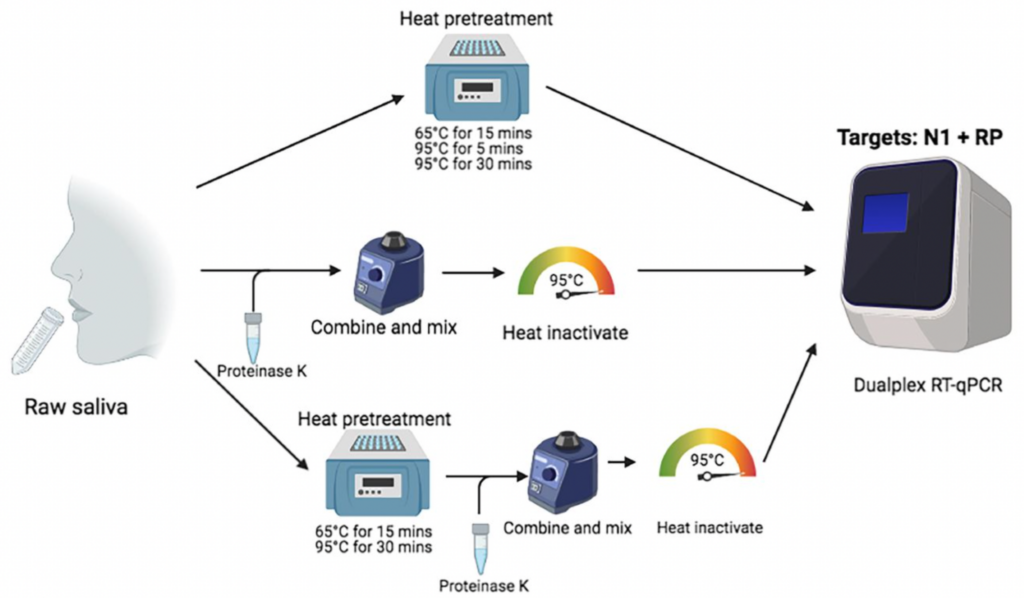
The SalivaDirect™ protocol utilizes a nucleic acid extraction-free workflow paired with real-time reverse transcription polymerase chain reaction (RT-PCR) technology to detect SARS-CoV-2 RNA in saliva. The process involves the following steps:
Patients provide a saliva sample by passively drooling into a sterile container or collection tube. Patients are advised not to eat or drink for 30 minutes before sample collection. Saliva self-collection instructions can be accessed here.
The SalivaDirect™ protocol has been validated for use in three different nucleic acid extraction-free sample preparation workflows; these workflows utilize a combination of Proteinase K and/or heat pre-treatments. These treatments break down proteins in the saliva and help release viral RNA, thereby removing the need for lengthy and costly RNA extraction steps. Labs are able to use the workflow that best integrates with their operations.
After sample preparation, the sample is analyzed using one-step RT-PCR amplification to detect any SARS-CoV-2 RNA present. Fluorescently labeled primers and probes from various vendors have been evaluated and selected to ensure optimal sensitivity and specificity in detecting viral RNA. Several PCR instruments have also been validated. See the vendors below.
Technical Requirements
Sample Collection
The SalivaDirect™ protocol has received EUA approval for three saliva sample collection methods:
- In the presence of a trained observer at a Designated Laboratory
- At home using the SalivaDirect™ At-home Collection Kit
- At home using the SalivaDirect™ Unsupervised Collection Kit
Reagents and Instruments
The SalivaDirect™ protocol has been validated for a wide range of reagents and instruments:
Reagents
|
Reagent |
Vendor |
Item |
Catalog Number(s) |
|
Proteinase K |
ThermoFisher Scientific |
MagMAX Viral/Pathogen Proteinase K |
A42363 |
|
New England Biolabs |
Proteinase K |
P8107S |
|
|
AmericanBio |
Proteinase K |
AB00925 |
|
|
RT-qPCR Kit |
New England Biolabs |
Luna Universal Probe One-Step RT qPCR (2x) Kit |
E3006S, E3006L, E3006X, E3006E |
|
Luna Probe One-Step RT-qPCR 4x Mix with UDG |
M3019S, M3019L, M3019X, M3019E |
||
|
Bio-Rad |
Reliance One-Step Multiplex RT-qPCR Supermix |
12010176, 12010220, 12010221 |
|
|
Quantabio |
UltraPlex 1-Step ToughMix |
95166-100, 95166-500, 95166-01K |
|
|
ThermoFisher Scientific |
TaqPath 1-Step RT-qPCR Master Mix, GC |
A15299, A15300 |
|
|
Primer/Probes |
Eurofins Genomics |
SalivaDirect™ primer and probe set |
12YS-010YST (Cy5), 12YS-010YS3 (HEX), 12YS-010YS2 (FAM) |
|
Lighthouse Lab Services |
SalivaNow SARS-CoV-2 Assay (primers/probes pre-mixed) |
9731816-S |
|
|
Integrated DNA Technologies |
nCOV_N1 Forward Primer |
10006821, 10006830 |
|
|
nCOV_N1 Reverse Primer |
10006822, 10006831 |
||
|
nCOV_N1 Probe |
10006823, 10006832 |
||
|
RNase P Forward Primer |
10006827, 10006836 |
||
|
RNase P Reverse Primer |
10006828, 10006837 |
||
|
RNase P Probe |
Custom order (Cy5) 10007061, 10007062 (ATTO647) |
||
|
Nuclease-free water |
Integrated DNA Technologies |
Nuclease-free water |
11-04-02-01, 11-05-01-14, 11-05-01-04 |
|
New England Biolabs |
Nuclease-free water |
B1500S, B1500L |
|
|
Controls |
Twist Bioscience |
Synthetic SARS-CoV-2 RNA Control 2 |
102024 |
|
Lighthouse Lab Services |
Positive CoV-2 Control |
9731816PC |
|
|
Negative Control |
9731816EC |
For a more comprehensive list of all reagents and instruments, review the full EUA, available at FDA.gov.
Instruments
|
Vendor |
Model(s) |
|
Bio-Rad |
CFX96 Touch, CFX384 Touch, CFX Opus Real-Time PCR Detection System |
|
ThermoFisher |
ABI StepOne/StepOne Plus |
|
Agilent |
AriaMX Real-Time PCR System |
|
Ubiquitome |
Liberty 16, Liberty 16 Pro |
|
Analytik Jena |
qTower |
|
Roche |
Cobas Z480, LightCycler 480 |
|
CHAI |
Open qPCR |
|
Bio Molecular Systems |
Mic, Mic qPCR/Q |
|
OnsiteGene |
XDiveTM Superfast Real-Time PCR System |
For a more comprehensive list of all instruments and validated software versions, review the full EUA, available at FDA.gov.
Workflows
The SalivaDirect™ protocol has been validated for use in three different nucleic acid extraction-free sample preparation workflows. See the image below:
SalivaDirect, Inc. is actively working toward obtaining FDA De Novo approval for the SalivaDirect™ SARS-CoV-2 assay. The protocol will initially include a single workflow using Proteinase K and focus on the following reagents and instruments:
|
Reagent |
Vendor |
Item |
Catalog Number(s) |
|
Proteinase K |
ThermoFisher Scientific |
MagMAX Viral/Pathogen Proteinase K |
A42363 |
|
RT-qPCR Kit |
New England Biolabs |
Luna Probe One-Step RT-qPCR 4x Mix with UDG |
M3019S, M3019L, M3019X, M3019E |
|
Primer/Probes |
Integrated DNA Technologies |
nCOV_N1 Forward Primer |
10006821, 10006830 |
|
nCOV_N1 Reverse Primer |
10006822, 10006831 |
||
|
nCOV_N1 Probe |
10006823, 10006832 |
||
|
RNase P Forward Primer |
10006827, 10006836 |
||
|
RNase P Reverse Primer |
10006828, 10006837 |
||
|
RNase P Probe |
Custom order (Cy5) 10007061, 10007062 (ATTO647) |
||
|
Nuclease-free water |
New England Biolabs |
Nuclease-free water |
B1500S, B1500L |
|
Controls |
Twist Bioscience |
Synthetic SARS-CoV-2 RNA Control 2 |
102024 |
|
Vendor |
Model(s) |
|
Bio-Rad |
CFX96 Touch Real-Time PCR Detection System |
|
ThermoFisher |
ABI QuantStudio 6 Pro Real-Time PCR System |
In Development: SalivaDirect+™
While the SalivaDirect™ protocol focuses exclusively on detecting SARS-CoV-2, SalivaDirect+™ is in development. Utilizing the SalivaDirect™ extraction-free protocol, SalivaDirect+™ will be able to detect SARS-CoV-2, Influenza A, Influenza B, and Respiratory Syncytial Virus (RSV) in a single saliva sample. By combining the detection of multiple viruses, SalivaDirect+™ will provide a more comprehensive tool for diagnosing and differentiating between various respiratory infections – ensuring providers and patients are empowered with comprehensive health information, reducing the use of inappropriate antibiotic treatment, and improving infectious disease surveillance data for public health agencies.
SalivaDirect, Inc. is currently conducting a prospective clinical validation study for the SalivaDirect+™ protocol. If you or your laboratory are interested in joining the study and supporting our assay development work, contact us at [email protected].
SalivaDirect, Inc. Publications
2024
- Contact with young children is a major risk factor for pneumococcal colonization in older adults (Wyllie et al., 2024)
- Detection of pneumococcal carriage in asymptomatic healthcare workers (Waghela, et al., 2024)
- Diagnostic testing preferences can help inform future public health response efforts: Global insights from an international survey (Salzano et al., 2024)
- Mobilizing community-driven public health response: increasing access to diagnostic testing for underserved and uninsured individuals in Connecticut through lab-in-a-van partnerships (Choate et al., 2024)
- Scalable solutions for global health: the SalivaDirect model (Wyllie et al., 2024)
2023
- A low-cost culture- and DNA extraction-free method for the molecular detection of pneumococcal carriage in saliva (Peno et al., 2023)
- Expansion of a low-cost, saliva-based PCR test for the detection of mpox virus (Thomas et al., 2023)
- Exploring the potential of a saliva-based, RNA-extraction-free PCR test for the multiplexed detection of key respiratory pathogens (Allicock et al., 2023)
- Method versatility in RNA extraction-free PCR detection of SARS-CoV-2 in saliva samples (Allicock et al., 2023)
- Pooled RNA-extraction-free testing of saliva for the detection of SARS-CoV-2 (Allicock et al., 2023)
- The potential of saliva as an accessible and sensitive sample type for the detection of respiratory pathogens and host immunity (Laxton et. al, 2023)
2022
- COVID Testing in the Workplace: Return to Work Testing in an Occupational Cohort (Sikka et al., 2022)
- Discordant SARS-CoV-2 PCR and Rapid Antigen Test Results When Infectious: A December 2021 Occupational Case Series (Sikka et al., 2022)
- Evaluation of saliva self-collection devices for SARS-CoV-2 diagnostics (Allicock et al., 2022)
- Persistence of pneumococcal carriage among older adults in the community despite COVID-19 mitigation measures (Wyllie et al., 2022)
- Routine Saliva Testing for SARS-CoV-2 in Children: Partnering with Childcare Centers in the Greater New Haven Community (Rayack et al., 2022)
- Saliva as a sample type for SARS-CoV-2 detection: implementation successes and opportunities around the globe (Tobik et al., 2022)
- Saliva-based methods for SARS-CoV-2 testing in low- and middle-income countries (Tan et al., 2022)
2021
- Saliva as a gold-standard sample for SARS-CoV-2 detection (Tan et al., 2021)
- Sequencing SARS-CoV-2 Genomes from Saliva (Alpert et al., 2021)
- Stability of SARS-CoV-2 RNA in Non Supplemented Saliva (Ott et al., 2021)
