Our Work
How SalivaDirect, Inc. is
Helping Communities
SalivaDirect, Inc. (SDI) is recognized internationally for developing accessible, adaptable, cost-effective testing solutions that can efficiently scale in response to evolving public health needs. We have also built a reputation of increasing access to testing through community programs. This includes deploying a mobile lab-in-a-van to neighborhoods in Connecticut, offering testing in rural Oregon via vending machines, and supporting community-driven capacity building in Malawi.
Throughout all of our on-the-ground initiatives, we work to establish community-focused programs, ensuring that patient perspectives and stakeholder input inform each stage of the diagnostic testing process, outreach strategy, and overall program design.
Looking to Set Up a Testing Program?
Check out the map below to view labs authorized to offer SalivaDirect™ testing. Contact the local lab directly regarding testing opportunities in your area.
Don’t see a local lab in your area? Contact the SDI team here.
Are you a lab interested in joining our network? Complete our brief inquiry form.
Our Programs
SalivaDirect, Inc. is a public health nonprofit with a mission to respond to global health challenges through development and deployment of diagnostic testing strategies.
SDI is recognized internationally for:
- Championing accessible and adaptable diagnostic testing solutions
- Developing low-cost, flexible qPCR assays using saliva
- Increasing access to testing through innovative community programs
- Training the next generation of public health professionals
- Building the workforce capacity of laboratories in low-resource settings
SalivaDirect, Inc. has disrupted the diagnostic industry with our innovative regulatory approach. Other diagnostic companies create a proprietary set of reagents to be run on their PCR instruments. SalivaDirect, Inc. has taken a different approach – creating the diagnostics industry’s equivalent to a “generic” diagnostic test.
Our flexible model allows laboratories to select from a number of reagents and PCR suppliers, which have been validated for use with SalivaDirect™. The SalivaDirect™ protocol can be run at a fraction of the cost of a traditional PCR test, allowing our labs to stretch resources further and scale up community-based testing programs.
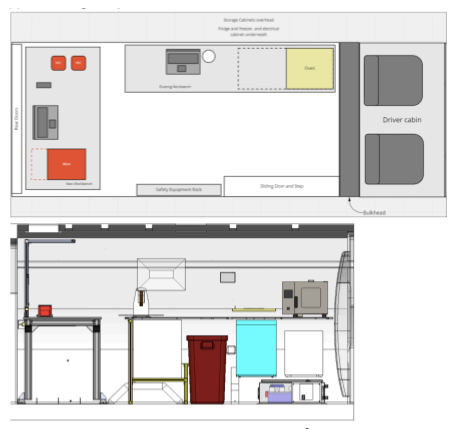
Mobile Laboratories
SalivaDirect, Inc. has piloted the use of a mobile laboratory to remove barriers and provide high-quality testing directly to communities in need.
In partnership with Yale Pathology Laboratory and with support from the NIH-RADx Underserved Populations program (RADxUP RP2 #R0604), we outfitted a cargo van to provide on-site, high-complexity clinical diagnostic testing. Free SARS-CoV-2 testing (using SalivaDirect™’s protocol) was offered at 123 community events across Connecticut. Tests were completed on the CLIA-licensed van, which was operated under the Yale University School of Medicine’s Department of Pathology.
Between June 2023 to July 2024, 1,428 tests were provided to community members – more than 1,000 above the pilot’s initial goal. Participants largely agreed it was easy to access the van and felt comfortable using the van for testing. In fact, 29% received their first-ever COVID-19 test at the van.
This pilot demonstrated the positive impact community-informed mobile testing could have for overcoming barriers to accessing healthcare, and its potential to serve as a framework for managing and responding to future public health needs.
Our mobile-laboratory model is adaptable to meet different public health needs and can be deployed by hospitals, diagnostic laboratories, or government agencies. Please contact us if you wish to explore mobile-laboratory options for your organization.
The images above were taken at community partner site WHEAT Inc (West Haven, CT) during a testing event. Photo credits: Gift Amadi. Graphics represent the overall project timeline and participant survey responses on why they chose a location for testing.
SDI's Mobile Laboratory
- Equipped with PCR machines to conduct diagnostic testing
- Active CLIA license – a standard for the diagnostics industry
- Can be run with minimal staff (a lab tech and/or a community engagement lead)
Click here to view this poster presented at IDWeek 2024, summarizing the Connecticut pilot.
Capacity Building
When conducting surveillance or diagnostic testing efforts, low and middle-income countries (LMICs) often face distinct challenges in their limited access to high-complexity laboratories as well as the significant cost of testing.
In response to this, SalivaDirect, Inc. and the Wyllie Laboratory at the Yale School of Public Health (YSPH) partnered with Professor Kondwani Jambo of the Malawi Liverpool Wellcome Trust (MLW) Clinical Research Programme to investigate the use of SalivaDirect+™ as a low-cost option for sustainable surveillance testing.
Together, we applied for and were awarded a grant from Balvi.io to support in-country laboratory capacity-building and community testing for SARS-CoV-2, Respiratory Syncytial Virus (RSV), Influenza A, and Influenza B. This effort was embedded in an existing COVID-19 community surveillance study led by Dr. Jambo, allowing us to compare the use of saliva to the use of nasopharyngeal swabs. Since December 2023, scientists at the Malawi Liverpool Wellcome Clinical Research Programme have collected 2,000+ saliva samples and successfully detected SARS-CoV-2, RSV, Influenza A, and Influenza B. Results thus far show substantial agreement between paired saliva and nasopharyngeal samples, demonstrating how saliva-based testing can act as a suitable low-cost alternative to nasopharyngeal swabs.
The images below are from an in-person training hosted by SDI for the MLW team to strengthen their laboratory’s skills in salivary diagnostics. This project is ongoing, and the MLW Clinical Research Programme continues to use SalivaDirect+™, enhancing Malawi’s surveillance testing capacity for common respiratory viruses.
Click here to view a poster summarizing the preliminary analysis from this program.
Please contact us if your lab would benefit from a capacity building partnership with SalivaDirect, Inc.
Vending Machines
SalivaDirect, Inc. piloted the use of vending machines to distribute diagnostic test kits in partnership with Santiam Hospital SCoPE Lab (SCoPE) and SimpliChek, increasing access to COVID-19 PCR testing in rural Oregon. With support from the NIH-RADx Underserved Populations program (RADxUP RP2 #R0703), community members in Oregon were able to access on-demand PCR testing through vending machines. These kiosks were placed at five trusted community partner locations. To access testing, patients registered to receive a test kit, followed self-collection instructions and deposited the kit into the vending machine’s collection bin. Once the test kit was returned, SCoPE lab was notified and retrieved samples for testing.
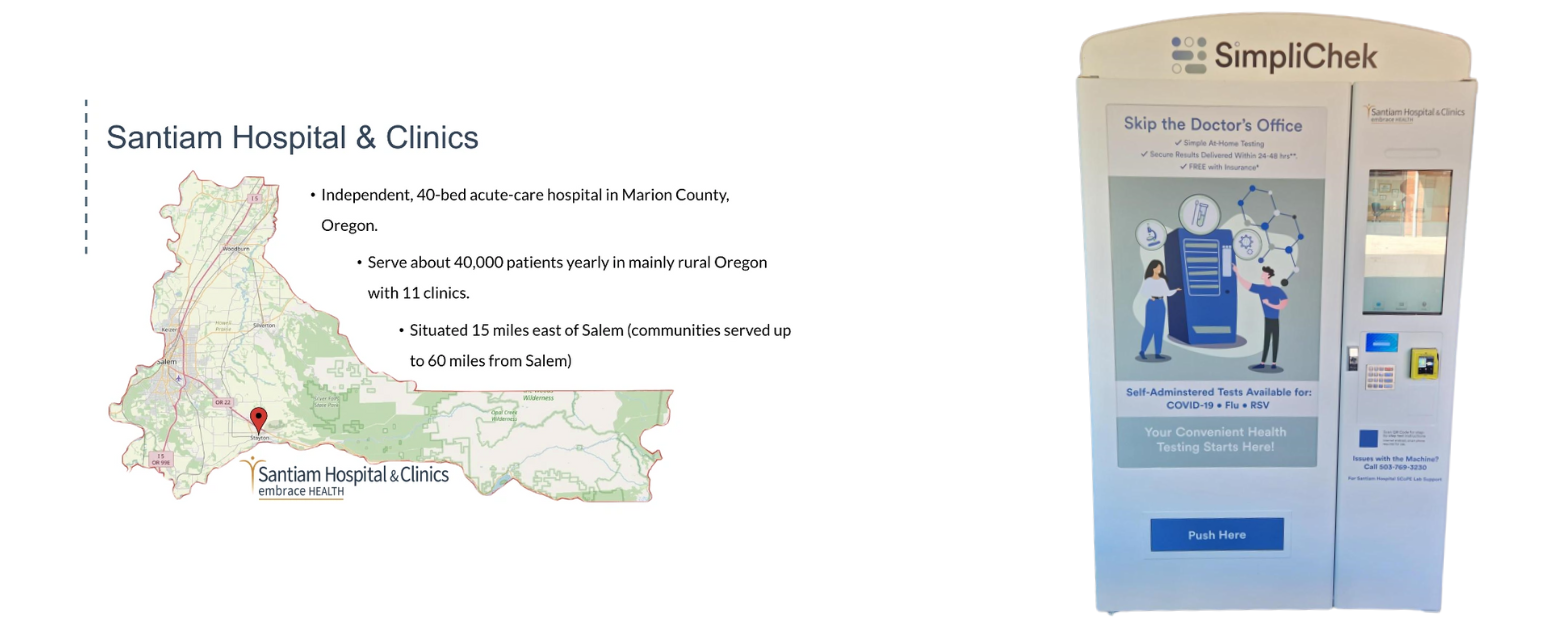
Above is a map of the areas served by this pilot; to the right is an image of the vending machines used to distribute testing kits in Oregon.
As a result of this pilot, SalivaDirect, Inc. submitted data to the U.S. Food and Drug Administration for Emergency Use Authorization of the vending machine distribution method.
Interested in learning more? Please contact us.
Toolkits
SalivaDirect, Inc. is committed to ensuring equitable access to diagnostic testing. We’ve documented our findings from different projects into toolkits for organizations to adopt and implement to fit their community needs. View and download our toolkits below.
SalivaDirect, Inc. Partners
SDI is proud to partner with organizations that share our commitment to delivering more affordable, equitable diagnostic testing. Together, we advance innovative solutions that respond to evolving public health challenges.
Interested in joining our network of SDI partners? Contact us at [email protected].
Research Partners
|
Name |
Website |
|
Malawi Liverpool Wellcome Trust |
|
|
Mirimus |
|
|
Santiam Hospital & Clinics SCoPE Lab |
|
|
Tempus |
|
|
Yale Pathology Labs |
|
|
Wichita State University |
|
|
Clemson University |
|
|
Pfizer |
Corporate Partners
|
Name |
Website |
|
Mirimus |
|
|
POREX Filtration Group |
|
|
Tempus |
|
|
Thermo Fisher Scientific |
|
|
SimpliChek |
U.S. Community Partners
|
Name |
Website |
|
Alliance for Living |
|
|
City of New Haven |
|
|
City of West Haven |
|
|
Clemson University |
|
|
COVID Safe Schools |
|
|
Fair Haven Community Health |
|
|
IRIS Connecticut |
|
|
Grubaugh Lab (Yale University) |
|
|
GS Biomark |
|
|
National Institute of Health (NIH) |
|
|
New Haven Health Department |
|
|
Santiam Hospital & Clinics SCoPE Lab |
|
|
SimpliChek |
|
|
RADx-UP (NIH) |
|
|
West Haven Health Department |
|
|
WHEAT, Inc. |
|
|
Yale Pathology Labs |
|
|
Yale School of Medicine |
International Partners
|
Name |
Website |
|
Balvi Filantropic Fund |
|
|
Malawi Liverpool Wellcome Trust |
|
|
Patient Centric Sampling Interest Group (PCSIG) |
Grant Sponsors
|
Name |
Website |
|
Balvi Filantropic Fund |
|
|
Fast Grants |
|
|
Flambeau RapidX |
|
|
Lighthouse Lab Services |
|
|
National Basketball Association (NBA) |
|
|
National Institute of Health (NIH) |
|
|
RADx-UP (NIH) |
|
|
RF Catalytic Capital (Rockefeller Foundation) |
|
|
Tempus |
|
|
Veritas Genetics |
Previous SDI Conference Sponsors
|
Name |
Website |
|
Co-Dx |
|
|
Flambeau RapidX |
|
|
GS Biomark |
|
|
Healthbit |
|
|
New England Biolabs (NEB) |
|
|
POREX Filtration Group |
|
|
Santiam Hospital & Clinics SCoPE Lab |
|
|
SimpliChek |
|
|
Seegene Inc |
|
|
SHIELD (University of Illinois) |
|
|
Tempus |
|
|
Thermo Fisher Scientific |
|
|
Ubiquitome |
|
|
Wichita State University |
